Polymers are generally obtained by polymerization, and the main constituent elements are C, H, O, N, and elements such as S, P, SI, and F also exist in some polymers.
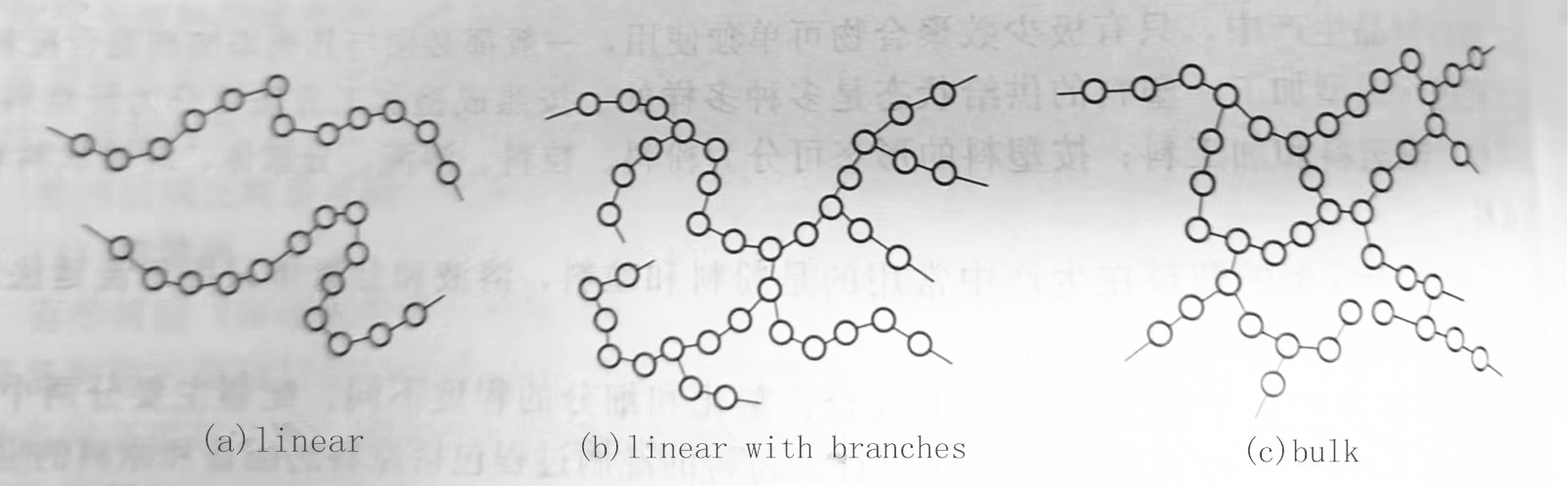
The molecular structure of polymers comes in three forms: linear, linear with branches, and bulk. If the molecular chain of the polymer is irregular linear (or agglomerate), and the polymer is composed of one molecular chain, it is called a linear polymer, as shown in Figure 1-1(a). The macromolecular main chain of the polymer has some long or short small branched chains, and the whole molecular chain is branched, as shown in Figure 1-1(b), which is called a linear polymer with branched chains . If there are some short chains between the chains of macromolecules to link them to form a three-dimensional network structure, it is called a bulk polymer, as shown in Figure 1-1(c)
Molecular aggregation refers to the geometric arrangement of molecules at equilibrium. Under normal temperature and pressure, most polymers are solid, not gaseous. The arrangement of solid aggregate molecules has two forms: the area where the molecules are regularly and closely arranged is called the crystalline area; the area where the molecules are in a disordered state is called This is the amorphous region, as shown in Figure 1-2. According to the geometric characteristics of molecular arrangement, solid aggregates can be divided into two types: crystalline and amorphous (or non-crystalline).
Crystalline polymer is composed of crystalline region and amorphous region, and the mass percentage of crystalline region is called crystallinity. For example, the crystallinity of low-pressure polyethylene at room temperature is 85%-90%. Generally speaking, the structural symmetry of the polymer macromolecular chain is good, the side groups on the main chain are small in volume, and the intermolecular force is large, which is conducive to crystallization; otherwise, it is unfavorable for crystallization or cannot form a crystalline region. Crystallization occurs only in linear polymers and bulk polymers with little crosslinking.
Crystallization has a great influence on the properties of polymers. Due to the close aggregation of molecules caused by crystallization, the intermolecular force is enhanced, so the strength, hardness, stiffness and melting point, heat resistance and chemical resistance of the polymer are improved, but the properties related to chain motion are improved. Such as elasticity, elongation and impact strength are reduced.
For the structure of amorphous polymers, the molecular arrangement in a large distance range is disordered and intertwined with each other. Order in a small distance, that is, "disorder at a distance, order at a short distance". Due to the large number of crosslinks between the molecular chains, it is difficult to arrange the molecular chains in an orderly manner, so most of the bulk polymers are amorphous polymers.
The diversity of polymer aggregation state leads to the diversity of its molding process. The polymer aggregation state transition depends on the molecular structure of the polymer, the composition of the system, the force and the ambient temperature. When the polymer and its composition are fixed, the transition of the polymerization state is mainly related to the temperature. When the temperature changes, the mechanical behavior of the plastic changes, showing different physical states and mechanical properties. As shown in Figure 1-3, it is the curve of the relationship between the deformation degree and the temperature of the linear amorphous polymer and the completely linear crystalline polymer under constant pressure, which is also a thermodynamic curve.
Tb is called the embrittlement temperature of the polymer, which is the lowest temperature at which the polymer maintains the mechanical properties of the polymer.
Tg, called the glass transition temperature, is the critical temperature at which a polymer transitions from a glassy state to a highly elastic state (or vice versa).
Tf, called the viscous flow temperature, is the critical temperature at which an amorphous polymer changes from a highly elastic state to a viscous flow state (or vice versa).
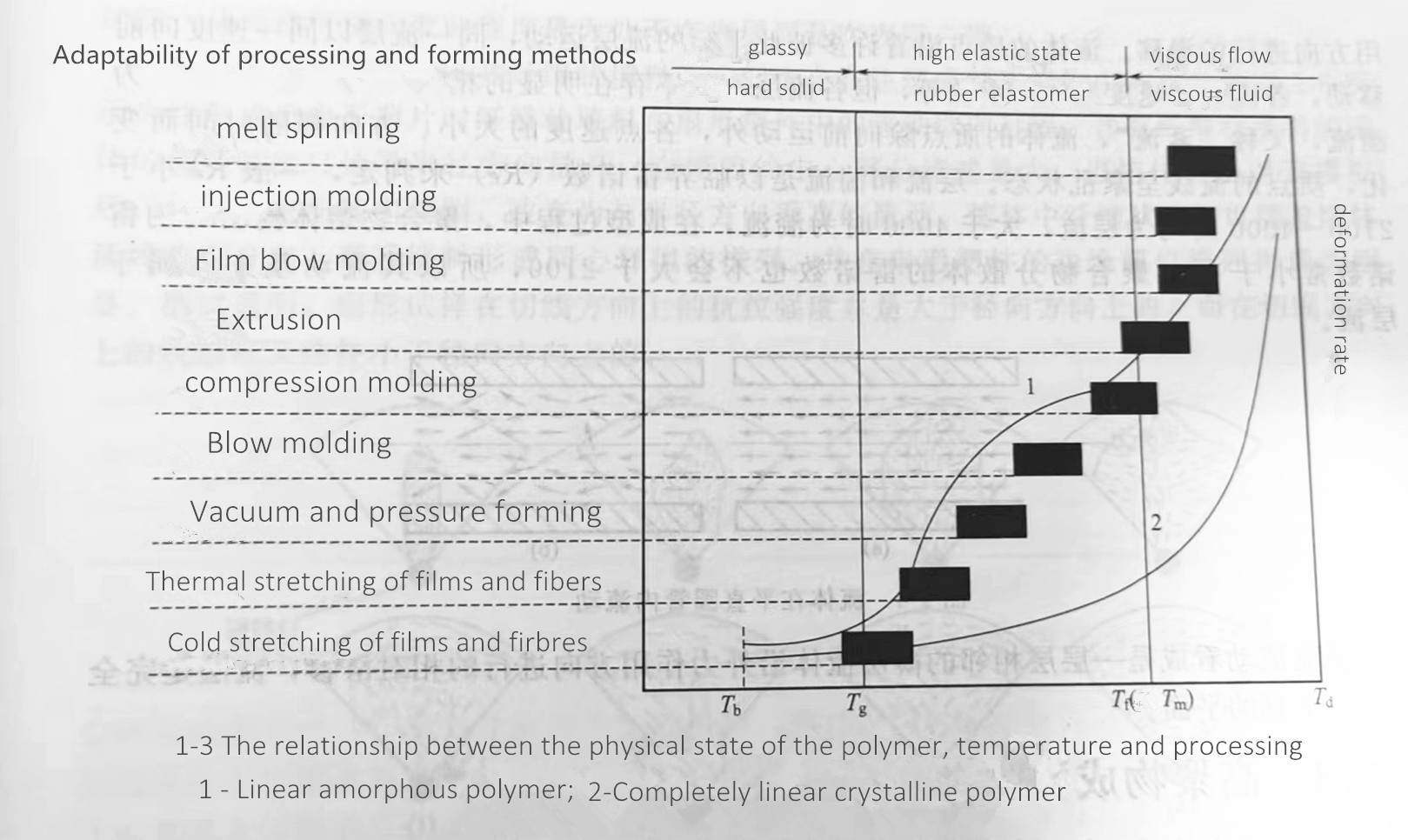
Tm, known as the melting point, is the temperature at which a crystalline polymer transitions from a crystalline state to a molten state (or vice versa).
Td is called thermal decomposition temperature, which is the critical temperature at which the polymer main chain breaks and starts to decompose when the polymer is heated to a certain temperature.
Rongdar Packing
www.rongdar.com
Rongdar (China )
E-mail:info@rongdar.com
Phone:8615000928169
Whatsapp:8615000928169
www.rongdar.com
Add: Cangzhou City, Hebei province,China .